CERVICAL
For surgeons
Clinical Studies
January 10, 2024
CAVUX FFS-LX Achieved 96% Fusion Rate Verified by Independent Core Imaging Lab in Recent Clinical Study
Pleasanton CA, January 10, 2024 / PRNewswire/ -- Providence Medical Technology, Inc. (PMT), a medical device innovator focused on improving surgical outcomes for high-risk spine surgery patients, announced that the U.S. Food and Drug Administration (FDA) has cleared its CAVUX® FFS-LX: Lumbar Facet Fixation System for use in lumbar spinal fusion surgery.
CAVUX® FFS-LX is a novel integrated cage and screw system that is implanted bilaterally in the facet joints to treat lumbar degenerative disc disease (DDD). The implant spans the facet interspace with points of fixation at each end of the construct to offer additional stabilization for 1- or 2-level lumbar interbody fusion. CAVUX® FFS-LX may be used with or without pedicle screws and rods and is implanted using the company’s CORUS™ Spinal System-LX tissue-sparing access and spinal fusion system.
The clearance marks the company's expansion into the $2 billion lumbar spine market characterized by a higher surgical failure rate than in the cervical spine. Patients who fail to fuse after a lumbar spine fusion surgery face a substantial risk of added complications, suffering, and costly revision procedures. CAVUX® FFS-LX was designed to offer increased stabilization following lumbar fusion procedures in order to increase fusion rates and reduce future complications and reoperations.
The clearance was supported by clinical study data demonstrating a strong safety and efficacy profile. Summary clinical study data reviewed by the FDA in considering the clearance included:
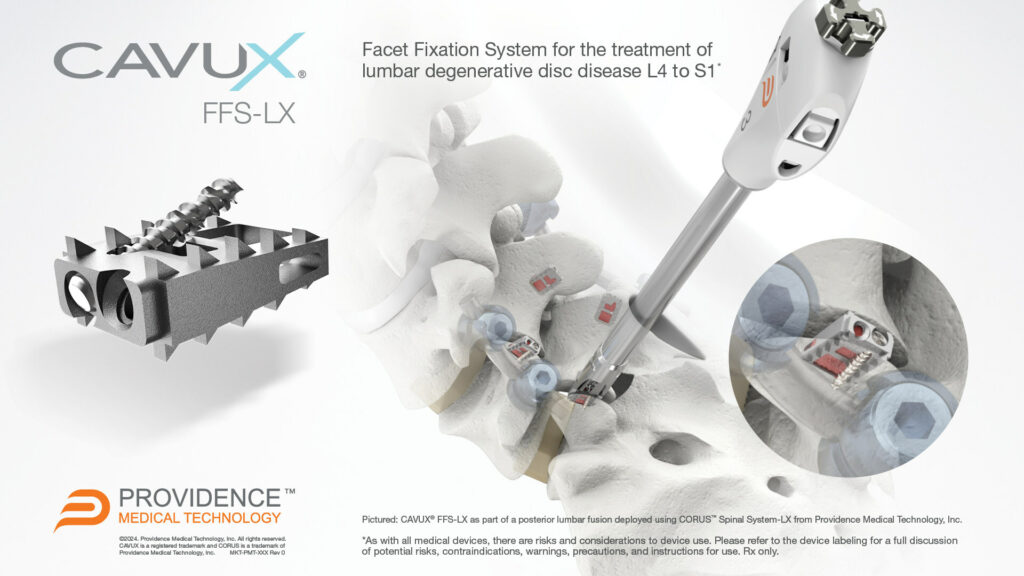
CAVUX® FFS-LX (Facet Fixation System, Lumbar) is a novel integrated cage and screw system to treat lumbar degenerative disc disease that is now FDA-cleared for use in lumbar spinal fusion surgery.
December 7, 2023
Latest Study Published in Journal of Clinical Neurosurgery adds to Robust Body of Evidence Supporting Tissue-Sparing Posterior Cervical Fusion to Treat High-Risk Cervical Fusion Patients
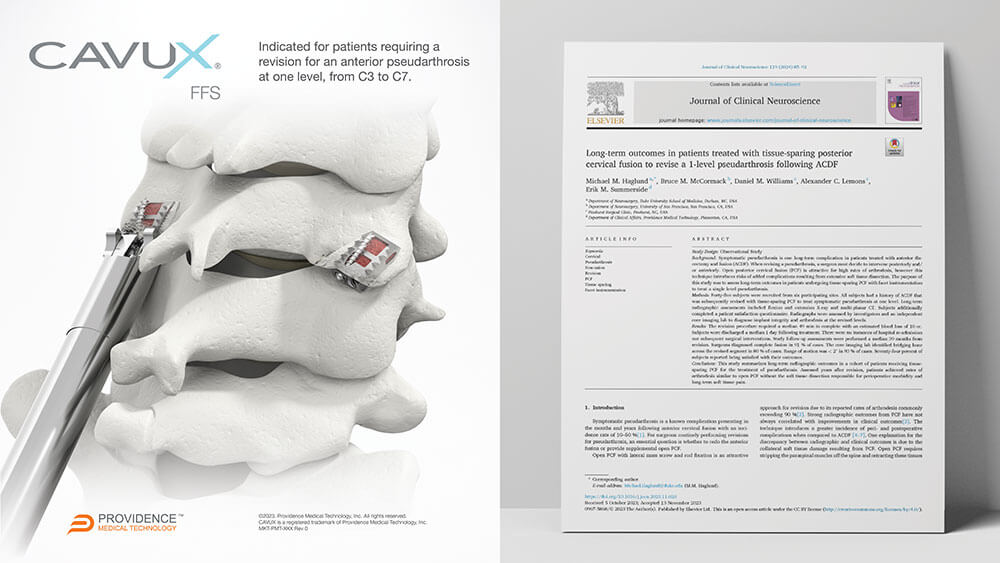
PLEASANTON, Calif., Dec. 7, 2023 /PRNewswire/ -- Providence Medical Technology, Inc., a medical device innovator focused on improving surgical outcomes for high-risk spine surgery patients, today announced a new publication in the Journal of Clinical Neuroscience. Dr. Michael M. Haglund and colleagues authored the publication on the long-term outcomes of patients undergoing tissue-sparing Posterior Cervical Fusion (PCF) to revise a 1-level pseudarthrosis following a failed Anterior Cervical Discectomy and Fusion (ACDF). View Release
May 11, 2023
PLEASANTON, Calif., May 11, 2023 /PRNewswire/ -- Providence Medical Technology, Inc. (PMT), a leader in medical device innovation for spine surgery, today announced the completion of enrollment in its FUSE Study — a prospective, multicenter, randomized, Investigational Device Exemption (IDE) clinical study evaluating the safety and effectiveness of its Posterior Cervical Stabilization System (PCSS) in 3-level cervical fusion patients. The study enrolled approximately 230 patients across 18 leading spinal surgery sites in the United States to evaluate PCSS in combination with Anterior Cervical Discectomy and Fusion (ACDF) compared to ACDF alone in a superiority study.
The study began in May 2020 and is designed to evaluate Circumferential Cervical Fusion (CCF) in high-risk cervical fusion patients by producing level-1 clinical evidence. The study includes patients at risk for failure of a standard ACDF fusion procedure due to risk factors such as 3-level disease, smoking, advanced age, and diabetes. While a traditional ACDF procedure is generally associated with positive outcomes in otherwise healthy patients, the success rate for these high-risk patients is significantly less favorable. Of the over 300,000 anterior cervical fusion procedures performed annually, roughly 30% involve patients with significant risk factors for nonunion. This study is investigating whether these patients could benefit from a tissue-sparing posterior cervical fusion as an adjunct to the ACDF.
The first comparative analysis for fusion and other clinical outcomes will occur when 100 patients reach two years of follow-up. The 100th patient is scheduled to return for their two-year follow-up visit at the end of 2023. View Release
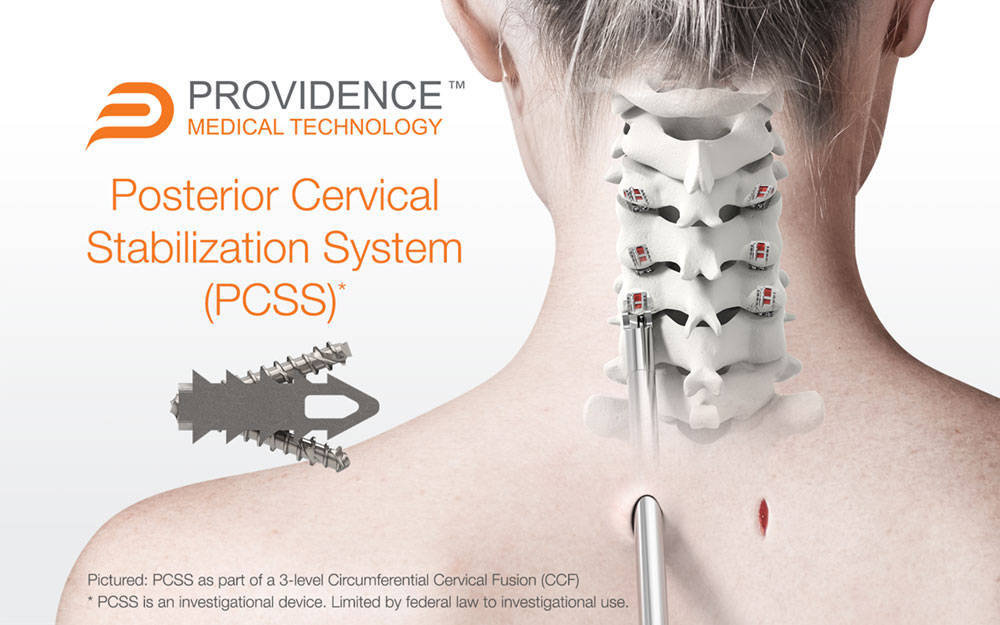
PCSS is an investigational implant system composed of non-segmental instrumentation with integrated screw fixation designed to provide immobilization and stabilization of spinal segments. PCSS is designed to achieve bilateral facet fixation at each level by spanning the interspace with points of fixation at each end of the construct.
December 15, 2022
Providence Medical Technology, Inc., a medical device innovator focused on improving surgical outcomes for high-risk spine surgery patients, announced that the U.S. Food and Drug Administration (FDA) has cleared its CAVUX® Facet Fixation System (FFS) for a new indication. The Facet Fixation System (FFS) is a novel integrated cage and screw system that is implanted bilaterally in the facet joints to provide stabilization until fusion occurs. View Release
Read MoreJune 15, 2020
Providence Medical Technology, Inc., a medical device innovator focused on improving surgical outcomes of the cervical spine, announced initiation of enrollment in a human clinical Investigational Device Exemption (IDE) study evaluating the safety and effectiveness of its Posterior Cervical Stabilization System (PCSS) in patients with 3-level degenerative disc disease. The first case was performed by Dr. Pierce Nunley, MD, Director of the Spine Institute of Louisiana in Shreveport, Louisiana.
September 20, 2018
Providence Medical Technology, Inc., an innovator in tissue-sparing surgical equipment and implants for cervical spine fusion surgery, today announced the closing of $25 million in new equity financing. The proceeds of the financing will be used to accelerate commercial expansion and clinical development of its DTRAX® line of cervical fusion instruments designed to help patients suffering from advanced cervical spine conditions.
May 22, 2018
Providence Medical Technology, Inc. today announced it has received 510(k) clearance from the United States Food and Drug Administration (FDA) for its DTRAX® Spinal System to be specifically indicated for use in posterior cervical fusion in patients with cervical degenerative disc disease.
January 8, 2018
Jeremy Laynor joins Providence as Vice President of US sales. Two new studies published in the Journal of Craniovertebral Junction and Spine further demonstrate the clinical benefits of Providence's unique cervical fusion technology.
September 27, 2017
Announces FDA Regulatory Clearance for ALLY® Posterior Fixation System.
July 10, 2017
Multiple independent studies demonstrate the results of Providence's tissue-sparing posterior cervical fusion technology.
