

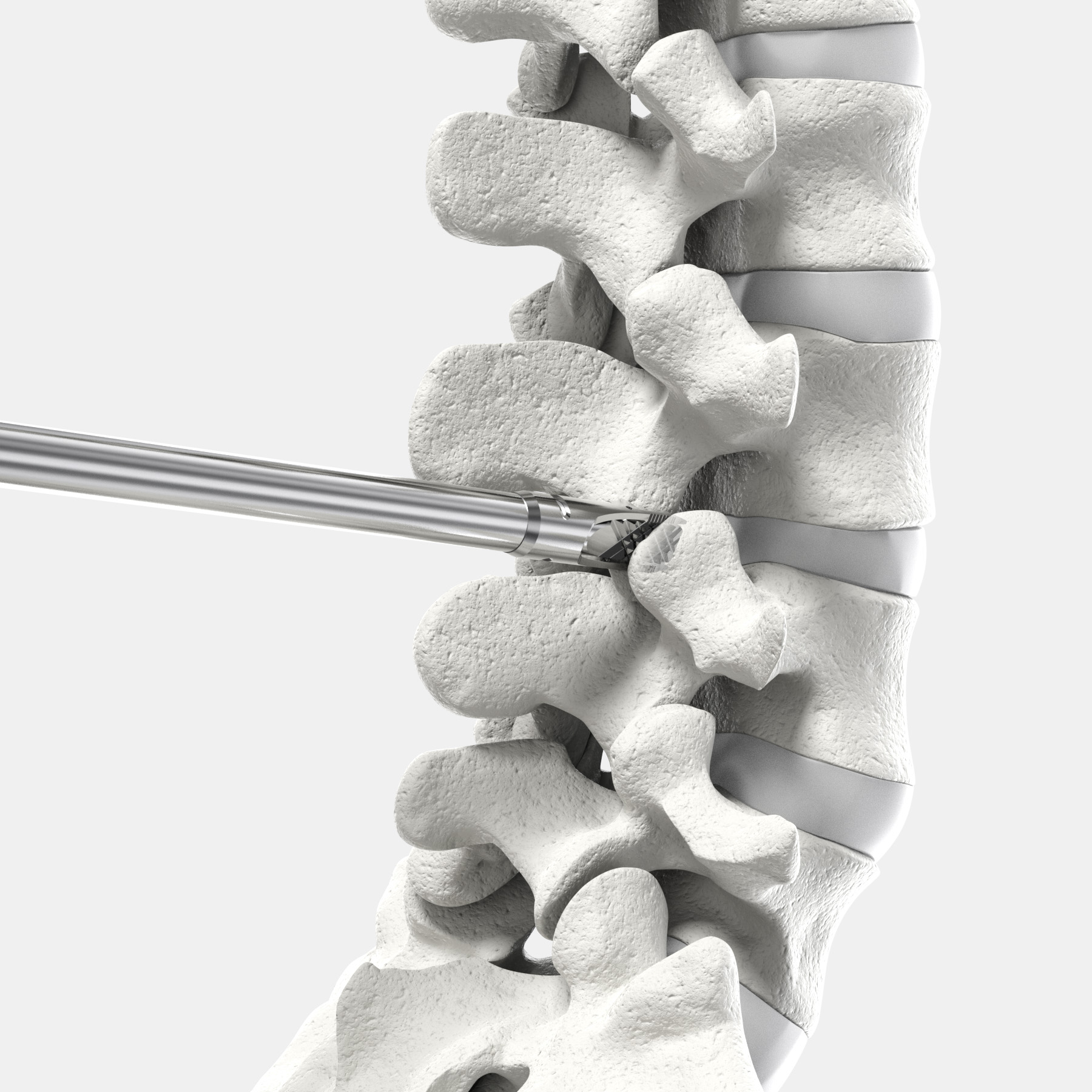
Posterior Lumbar Fusion Fusion with CORUS™ Spinal System-LX is designed to provide robust bone preparation and avoid muscle stripping.*
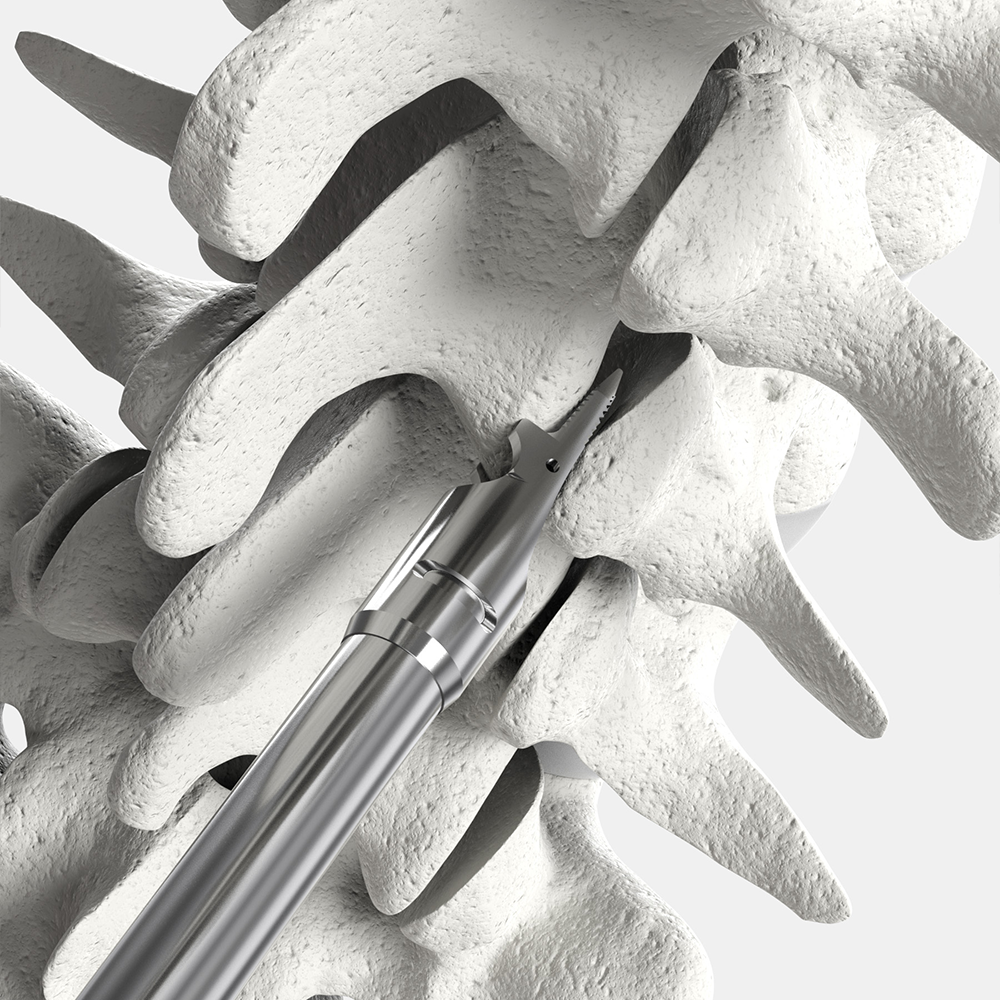
CORUS™ Spinal System-LX instruments feature positive stops for precise control and patient safety.


As with all medical devices, there are risks and considerations to device use. Please refer to the device labeling for a full discussion of potential risks, contraindications, warnings, precautions, and instructions for use.
* Posterior lumbar fusion can be performed using open or tissue-sparing techniques. Tissue-sparing technique may require special training, utilize indirect visualization, and/or increase radiation exposure.
MKT-PMT-597 Rev 0
Indications for Use, CORUS™ Spinal System-LX
The CORUS™ Spinal System-LX is a set of instruments indicated to be used to perform posterior lumbar fusion in patients with lumbar degenerative disc disease.
Device Description
The CORUS Spinal System-LX disposable instruments are used to access and prepare the posterior lumbar spine for joint fusion by decortication of bone surfaces, including the posterior lateral mass and facet joints, combined with application of allograft or autograft in patients with or without anterior or posterior instrumentation. It is recommended that commercially available autograft or allograft be used to aid fusion. Autograft or allograft material is not supplied as part of the system.
