
A. Schedule a Time with our Principal Scientist, Erik Summerside, PhD
B. Please have someone contact me
Not Ready to Connect?
We invite you to learn more about the technology under evaluation via a brief online course.
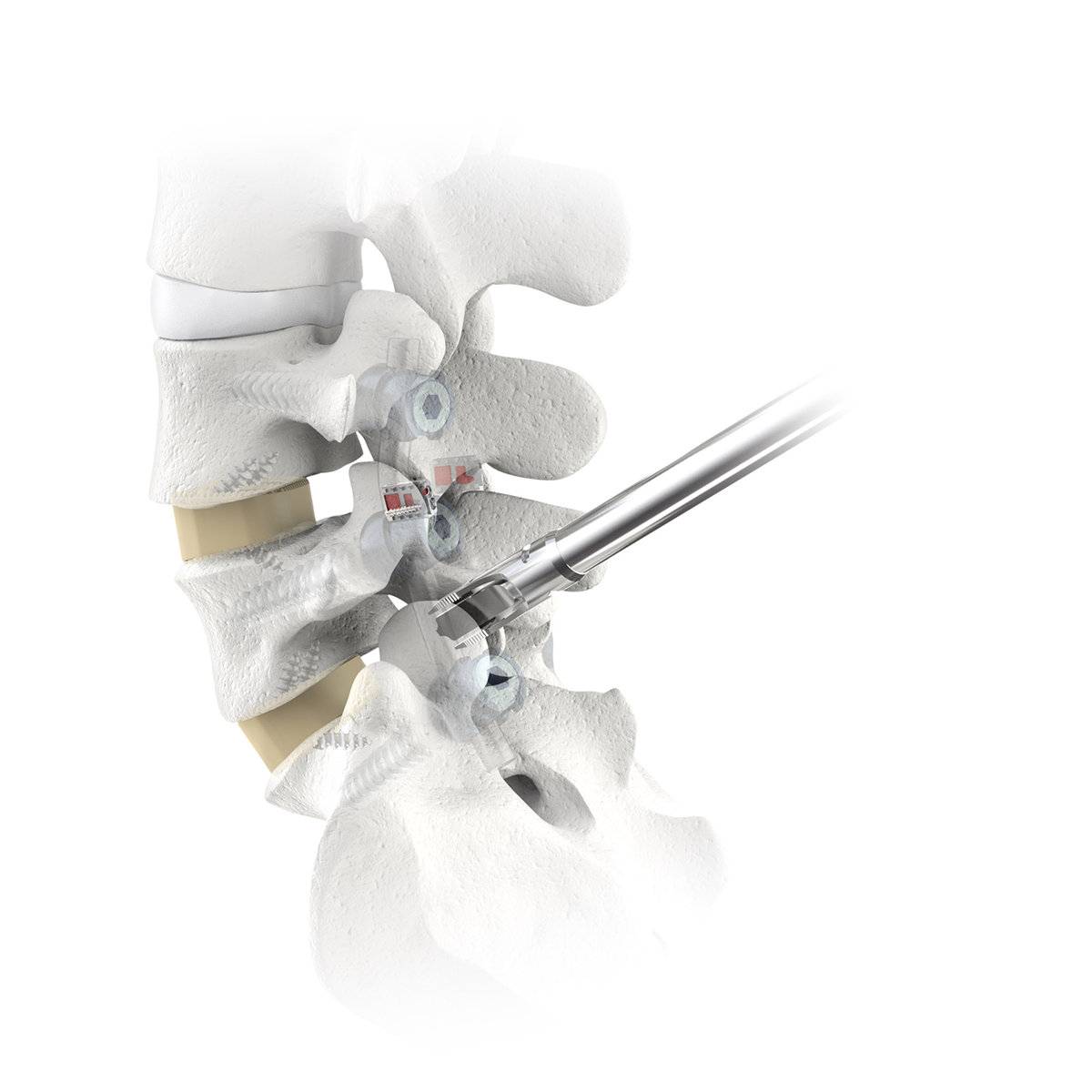
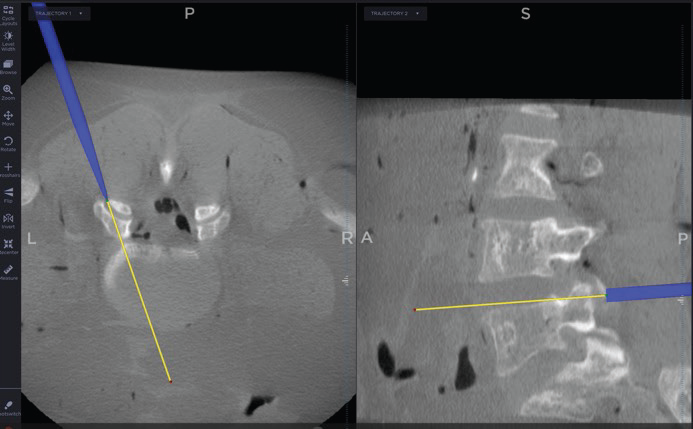
Access Course: Introduction to Navigated Lumbar Fusion with CORUS-LX
