CERVICAL
For surgeons
Clinical Studies
09/05/2024
Podium presentation by: Dr. Bruce M. McCormack, MD
Title: Twenty-four month assessment of arthrodesis following ACDF at three contiguous levels from subjects enrolled prospectively in the FUSE IDE Clinical Trial
Session Date & Time: Friday, September 6 from 10:53:00 AM-10:57:00 AM
Read More12/11/2024
Cervical Spine Research Society
Accepted Podium Presentation: Impact of added morbidity when performing cervical circumferential cervical fusion (CCF) compared to ACDF alone: results from 227 prospectively randomized enrolled subjects treated with 3-level fusion
Accepted Poster: Twenty-four-month assessment of arthrodesis in a prospectively enrolled cohort of 50 subjects treated with ACDF at 3 levels
Read MoreJuly 11, 2024
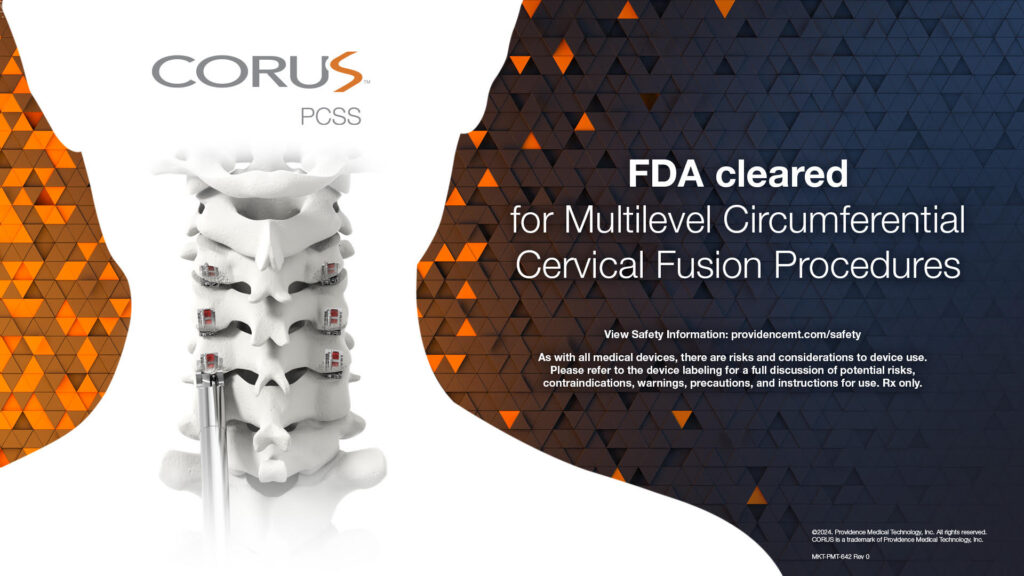
FDA clears CORUS™ PCSS for multilevel circumferential cervical fusion procedures based on results from the prospective, multicenter, randomized, controlled FUSE Study
PLEASANTON, Calif., July 11, 2024 /PRNewswire/ -- Providence Medical Technology announces FDA Clearance of its CORUS™ Posterior Cervical Stabilization System (PCSS) for the treatment of up to 3-level cervical Degenerative Disc Disease (DDD). The FDA clearance was based on results from the FUSE IDE study, a prospective, multicenter, randomized controlled trial comparing safety and effectiveness of Circumferential Cervical Fusion (CCF) versus Anterior Cervical Discectomy and Fusion (ACDF) alone in high-risk cervical fusion patients.
The FUSE Study was performed at 18 U.S. sites across 13 states and enrolled 227 patients aged 18 to 80, with symptomatic cervical DDD at three contiguous levels. The study randomized subjects to receive ACDF, the current standard of care, or CCF (ACDF plus posterior cervical fusion with the company's PCSS technology). Interim analysis was performed on over 200 subjects at 1 year follow-up and 100 patients at 2 years follow-up.
The interim analysis demonstrated the superiority of CCF in the study's primary endpoint of fusion success, highlighting the profound benefits of tissue-sparing CCF over ACDF alone:
Superior Fusion Success at 1 year: The study's primary endpoint demonstrated a 44.3% higher composite fusion rate of CCF over ACDF (61.0% vs 16.7%, p<0.001) using a strict definition of composite fusion. Fusion was deemed a success if all three consecutive levels demonstrated motion of less than 2 degrees on flexion-extension radiographs and continuous bridging bone was exhibited across the endplates of all three segments on thin-slice CT scans. Results were reported by an independent core imaging lab and verified by multiple radiologists.
Dramatic Difference in Revision Rates: Across all ACDF subjects followed through study completion, 22.8% (13/57) required subsequent surgical intervention (primarily due to symptomatic nonunion), compared to 1.7% (1/59) in the CCF arm.
Overall Safety Success at 2 years: The study's secondary endpoint measured overall safety success at 2 years using a composite of fusion success, lack of subsequent surgical interventions, maintenance or improvement in neurological success, and Neck Disability Index (NDI) improvement. The CCF arm exhibited a superior composite overall safety success rate at 24 months compared to the ACDF arm (CCF=50.8%, ACDF=22.8%, p<0.002).
No Increase in procedure-related Adverse Events: 3-level CCF with PCSS demonstrated statistically lower procedure-related adverse events than 3-level ACDF (p=0.005).
These findings underscore the transformative potential of CCF with the PCSS device in managing degenerative disc disease in high-risk cervical fusion patients. With minimal added operative morbidity, CCF delivers significant improvement to long-term outcomes over the current standard of care. As such, results from this pivotal FUSE study represent a consequential advancement that redefines the standard of care for patients with multilevel disease.
Dr. Pierce D. Nunley, MD, the Director of the Spine Institute of Louisiana and a Principal Investigator in the FUSE Study, commented on the significance of the findings, stating, "The results from this study provide very compelling evidence for the use of circumferential cervical fusion with PCSS over the standard ACDF treatment for multilevel cervical degenerative disc disease. CCF patients had significantly higher fusion rates and greater improvements in patient-reported symptoms and overall quality of life. Furthermore, the Secondary Surgical Intervention (SSI) rates demonstrate the durability of CCF with PCSS: only 1.7% of CCF subjects (1/59) who completed the study required SSI, compared to 22.8% of ACDF subjects (13/57). This study should cause spine surgeons and payors to re-examine their preferred approach to treating 3-level cervical DDD."
Jeff Smith, Chief Executive Officer of Providence Medical Technology, remarked, "The findings from the FUSE study mark a milestone in spinal surgery. These outcomes unequivocally demonstrate that 3-level CCF with CORUS PCSS has superior efficacy for spinal fusion and that 3-level ACDF fusion rates are unacceptably low. The high rates of 3-level ACDF failures and reoperation underscore how these patients need more to heal properly and achieve positive outcomes. The strength of this clinical evidence suggests that CCF with CORUS PCSS should become the new standard of care for multilevel fusion patients."
For more information about the FUSE study and its implications, please visit: www.providencemt.com
The FUSE Clinical Study aims to redefine the standard of care for this prevalent condition through rigorous scientific inquiry and innovative treatment approaches. https://clinicaltrials.gov/study/NCT04229017
Indications for Use:
CORUS™ Posterior Cervical Stabilization System (PCSS) is posterior spinal instrumentation with integrated screw fixation intended to provide immobilization and stabilization of spinal segments.
CORUS PCSS is placed through a posterior surgical approach in up to 3 consecutive levels of the cervical spine (C3-C7) and achieves bilateral facet fixation by spanning the facet interspace at each level with points of fixation at each end of the construct.
CORUS PCSS is intended as an adjunct to posterior cervical fusion (PCF) and is only intended to be used in combination with an anterior cervical discectomy and fusion (ACDF) at the same level(s).
CORUS PCSS is indicated for skeletally mature patients with degenerative disc disease (DDD). DDD is defined as radiculopathy and/or myelopathy, neck and/or arm pain of discogenic origin as confirmed by radiographic studies.
CORUS PCSS is to be used with autogenous bone and/or allogenic bone graft.
SOURCE Providence Medical Technology, Inc.
June 13, 2024
Pleasanton, CA – June 13, 2024 – Providence Medical Technology, Inc., an innovator in solutions for spinal surgery, announces that the U.S. Food and Drug Administration (FDA) has cleared the use of the CORUS™ Navigation Access System for use with the CORUS Spinal System during spinal surgery. The CORUS Navigation Access System is specifically designed for use with Medtronic's StealthStation™ System for posterior spinal fusion procedures. This FDA clearance strengthens Providence Medical Technology's portfolio and underscores its commitment to advancing spinal surgery for high-risk spinal fusion patients.
The CORUS™ Navigation Access System is intended to assist surgeons in locating, accessing, and preparing facet joints for posterior fusion procedures. It enables surgeons to utilize surgical navigation to perform procedures with its novel CORUS Spinal System. Surgical guidance and navigation have become increasingly utilized in spinal surgical procedures, particularly for less invasive surgical techniques.
The potential benefits of utilizing the CORUS™ Navigation Access System to perform a navigated CORUS fusion include:
Bryan Lee, MD, a neurosurgeon at Barrow Neurological Institute, commented, "The newly released CORUS navigation system offers simplicity and accuracy for placement of CAVUX® FFS-LX implants and provides the surgeon with a truly minimally invasive approach for posterior fusion, with a wide array of indications, including advanced disc degeneration and adjacent segment disease."
"We are thrilled to integrate our CORUS™ Spinal System with the leading surgical navigation platform and allow for incredible precision and safety in posterior fusion surgeries," said Jeff Smith, CEO of Providence Medical Technology. "This clearance signifies another leap forward in our mission to improve clinical outcomes for high-risk patients and prevent surgical failures of the spine.”
For more information about the CORUS™ Navigation Access System please visit providencemt.com.
As with all medical devices, there are risks and considerations to device use. Please refer to the device labeling for a full discussion of potential risks, contraindications, warnings, precautions, and instructions for use. Rx only. View product safety information: providencemt.com/safety
About Providence Medical Technology, Inc.
Providence Medical Technology, Inc. is a leading medical device company focused on advancing spine surgery technologies. Its commitment to improving clinical outcomes and reducing failures in high-risk spinal surgery has led PMT to develop a range of innovative solutions like CORUS™ Spinal Systems, CAVUX® Facet Fixation Systems, and ALLY® Bone Screws and Facet Screws.
Indications for use:
The CORUS™ Navigation Access System for use with the CORUS™ Spinal System is intended to be used during spinal surgery to assist the surgeon in locating and preparing facet joints in either open or minimally invasive procedures. The CORUS™ Navigation Access System is specifically designed for use with the Medtronic StealthStation™ System, which is indicated for any medical condition in which the use of stereotactic surgery may be appropriate, and where reference to a rigid anatomical structure, such as a long bone, or vertebra can be identified relative to a CT or MR-based model, fluoroscopy images, or digitized landmarks of the anatomy.
CORUS™ Spinal System-X is a set of instruments indicated to be used to perform posterior cervical fusion in patients with cervical degenerative disc disease.
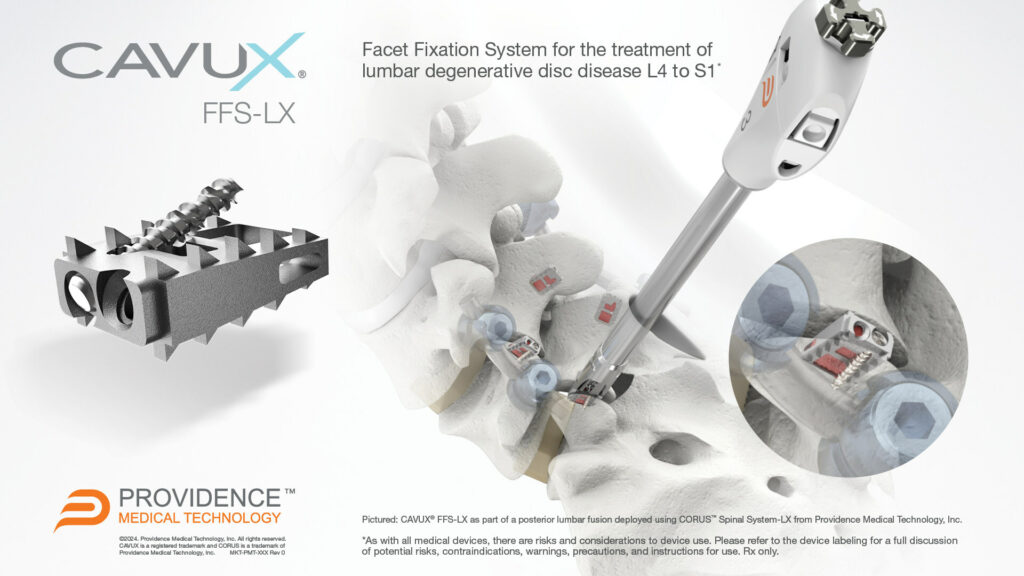
CORUS™ Spinal System-LX is a set of instruments indicated to be used to perform posterior lumbar fusion in patients with lumbar degenerative disc disease.
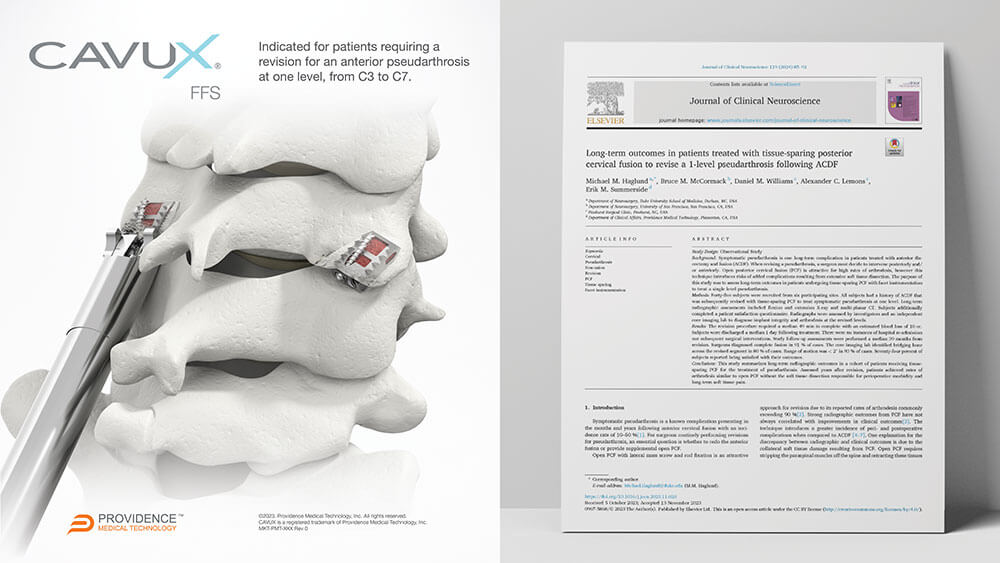
CAVUX® Facet Fixation System (CAVUX FFS) is an integrated construct comprised of a CAVUX Cage and a single ALLY Bone Screw. CAVUX FFS is placed bilaterally through a posterior surgical approach and spans the interspace with points of fixation at each end of the construct. CAVUX FFS is intended for temporary stabilization as an adjunct to posterior cervical fusion in skeletally mature patients. CAVUX FFS is indicated for patients requiring a revision for an anterior pseudarthrosis at one level, from C3 to C7, with autogenous and/or allogenic bone graft.
CAVUX Facet Fixation System, Lumbar (CAVUX FFS-LX) is an integrated construct comprised of a CAVUX Cage and an ALLY Bone Screw. CAVUX FFS-LX is placed bilaterally through a posterior surgical approach and spans the facet interspace with points of fixation at each end of the construct. CAVUX FFS-LX is intended to provide temporary stabilization as an adjunct to a 1 or 2 level interbody lumbar fusion with autogenous and/or allogenic bone graft and must be accompanied with an FDA-cleared intervertebral body fusion device implanted at the same spinal level(s), and may be used with a pedicle screw and rod system implanted at the same spinal level(s). CAVUX FFS-LX is indicated for the treatment of patients with lumbar degenerative disc disease (DDD) from L4 to S1 in skeletally mature patients who have failed conservative care.
SOURCE Providence Medical Technology, Inc.

January 10, 2024
CAVUX FFS-LX Achieved 96% Fusion Rate Verified by Independent Core Imaging Lab in Recent Clinical Study
Pleasanton CA, January 10, 2024 / PRNewswire/ -- Providence Medical Technology, Inc. (PMT), a medical device innovator focused on improving surgical outcomes for high-risk spine surgery patients, announced that the U.S. Food and Drug Administration (FDA) has cleared its CAVUX® FFS-LX: Lumbar Facet Fixation System for use in lumbar spinal fusion surgery.
CAVUX® FFS-LX is a novel integrated cage and screw system that is implanted bilaterally in the facet joints to treat lumbar degenerative disc disease (DDD). The implant spans the facet interspace with points of fixation at each end of the construct to offer additional stabilization for 1- or 2-level lumbar interbody fusion. CAVUX® FFS-LX may be used with or without pedicle screws and rods and is implanted using the company’s CORUS™ Spinal System-LX tissue-sparing access and spinal fusion system.
The clearance marks the company's expansion into the $2 billion lumbar spine market characterized by a higher surgical failure rate than in the cervical spine. Patients who fail to fuse after a lumbar spine fusion surgery face a substantial risk of added complications, suffering, and costly revision procedures. CAVUX® FFS-LX was designed to offer increased stabilization following lumbar fusion procedures in order to increase fusion rates and reduce future complications and reoperations.
The clearance was supported by clinical study data demonstrating a strong safety and efficacy profile. Summary clinical study data reviewed by the FDA in considering the clearance included:
CAVUX® FFS-LX (Facet Fixation System, Lumbar) is a novel integrated cage and screw system to treat lumbar degenerative disc disease that is now FDA-cleared for use in lumbar spinal fusion surgery.
December 7, 2023
Latest Study Published in Journal of Clinical Neurosurgery adds to Robust Body of Evidence Supporting Tissue-Sparing Posterior Cervical Fusion to Treat High-Risk Cervical Fusion Patients
PLEASANTON, Calif., Dec. 7, 2023 /PRNewswire/ -- Providence Medical Technology, Inc., a medical device innovator focused on improving surgical outcomes for high-risk spine surgery patients, today announced a new publication in the Journal of Clinical Neuroscience. Dr. Michael M. Haglund and colleagues authored the publication on the long-term outcomes of patients undergoing tissue-sparing Posterior Cervical Fusion (PCF) to revise a 1-level pseudarthrosis following a failed Anterior Cervical Discectomy and Fusion (ACDF). View Release
May 11, 2023
PLEASANTON, Calif., May 11, 2023 /PRNewswire/ -- Providence Medical Technology, Inc. (PMT), a leader in medical device innovation for spine surgery, today announced the completion of enrollment in its FUSE Study — a prospective, multicenter, randomized, Investigational Device Exemption (IDE) clinical study evaluating the safety and effectiveness of its Posterior Cervical Stabilization System (PCSS) in 3-level cervical fusion patients. The study enrolled approximately 230 patients across 18 leading spinal surgery sites in the United States to evaluate PCSS in combination with Anterior Cervical Discectomy and Fusion (ACDF) compared to ACDF alone in a superiority study.
The study began in May 2020 and is designed to evaluate Circumferential Cervical Fusion (CCF) in high-risk cervical fusion patients by producing level-1 clinical evidence. The study includes patients at risk for failure of a standard ACDF fusion procedure due to risk factors such as 3-level disease, smoking, advanced age, and diabetes. While a traditional ACDF procedure is generally associated with positive outcomes in otherwise healthy patients, the success rate for these high-risk patients is significantly less favorable. Of the over 300,000 anterior cervical fusion procedures performed annually, roughly 30% involve patients with significant risk factors for nonunion. This study is investigating whether these patients could benefit from a tissue-sparing posterior cervical fusion as an adjunct to the ACDF.
The first comparative analysis for fusion and other clinical outcomes will occur when 100 patients reach two years of follow-up. The 100th patient is scheduled to return for their two-year follow-up visit at the end of 2023. View Release
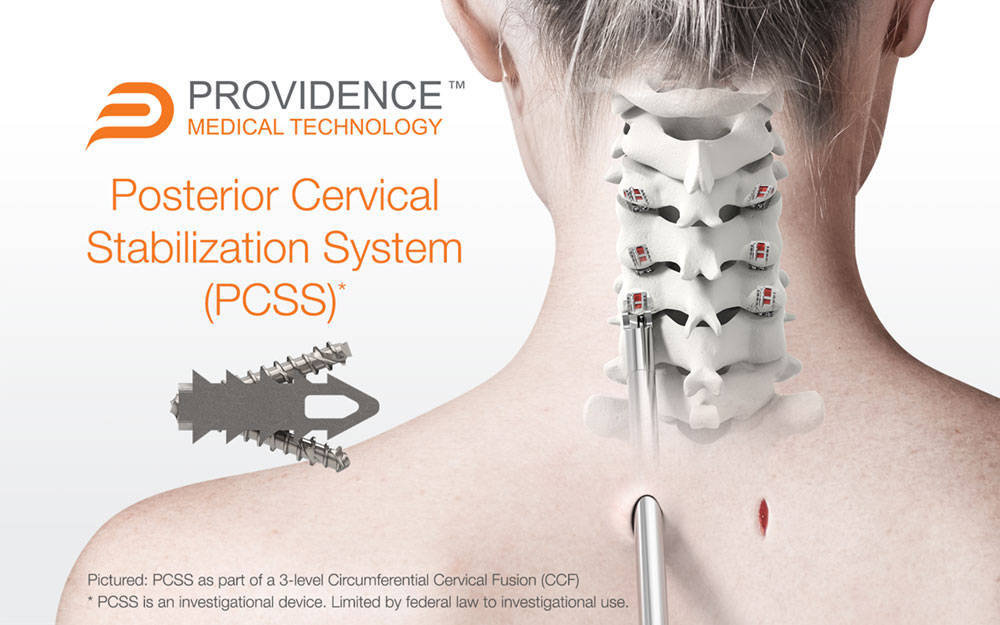
PCSS is an investigational implant system composed of non-segmental instrumentation with integrated screw fixation designed to provide immobilization and stabilization of spinal segments. PCSS is designed to achieve bilateral facet fixation at each level by spanning the interspace with points of fixation at each end of the construct.
December 15, 2022
Providence Medical Technology, Inc., a medical device innovator focused on improving surgical outcomes for high-risk spine surgery patients, announced that the U.S. Food and Drug Administration (FDA) has cleared its CAVUX® Facet Fixation System (FFS) for a new indication. The Facet Fixation System (FFS) is a novel integrated cage and screw system that is implanted bilaterally in the facet joints to provide stabilization until fusion occurs. View Release
Read MoreJune 15, 2020
Providence Medical Technology, Inc., a medical device innovator focused on improving surgical outcomes of the cervical spine, announced initiation of enrollment in a human clinical Investigational Device Exemption (IDE) study evaluating the safety and effectiveness of its Posterior Cervical Stabilization System (PCSS) in patients with 3-level degenerative disc disease. The first case was performed by Dr. Pierce Nunley, MD, Director of the Spine Institute of Louisiana in Shreveport, Louisiana.
September 20, 2018
Providence Medical Technology, Inc., an innovator in tissue-sparing surgical equipment and implants for cervical spine fusion surgery, today announced the closing of $25 million in new equity financing. The proceeds of the financing will be used to accelerate commercial expansion and clinical development of its DTRAX® line of cervical fusion instruments designed to help patients suffering from advanced cervical spine conditions.
May 22, 2018
Providence Medical Technology, Inc. today announced it has received 510(k) clearance from the United States Food and Drug Administration (FDA) for its DTRAX® Spinal System to be specifically indicated for use in posterior cervical fusion in patients with cervical degenerative disc disease.
January 8, 2018
Jeremy Laynor joins Providence as Vice President of US sales. Two new studies published in the Journal of Craniovertebral Junction and Spine further demonstrate the clinical benefits of Providence's unique cervical fusion technology.
