CERVICAL
For surgeons
Clinical Studies

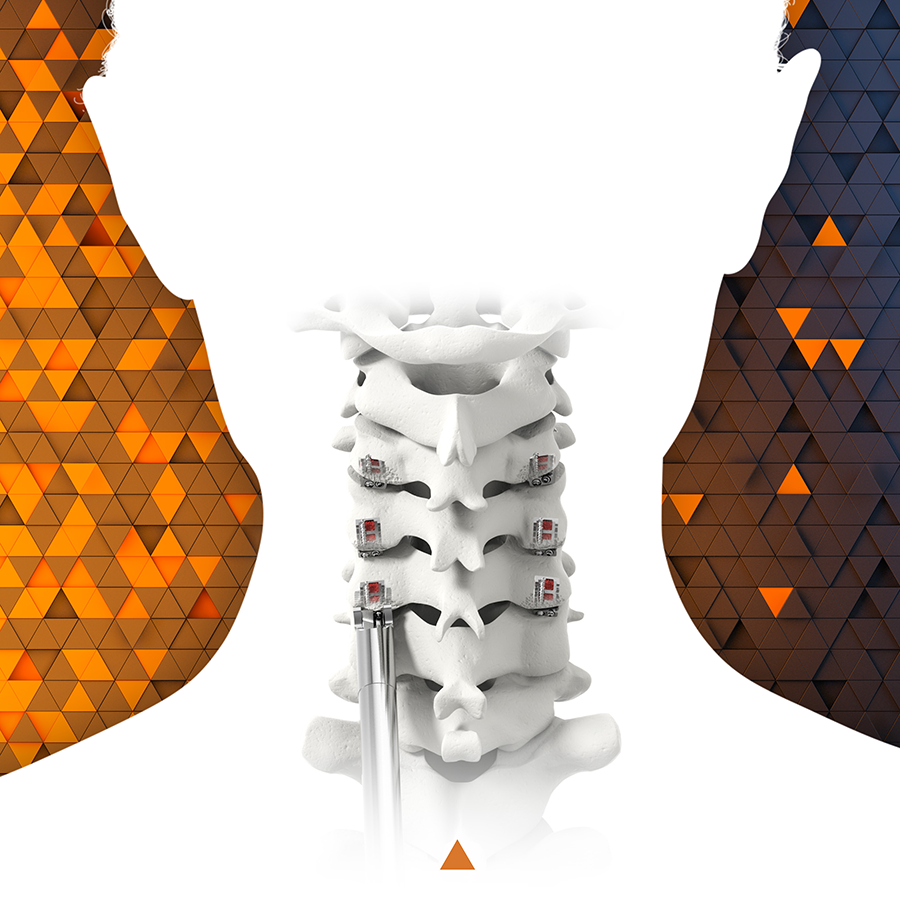
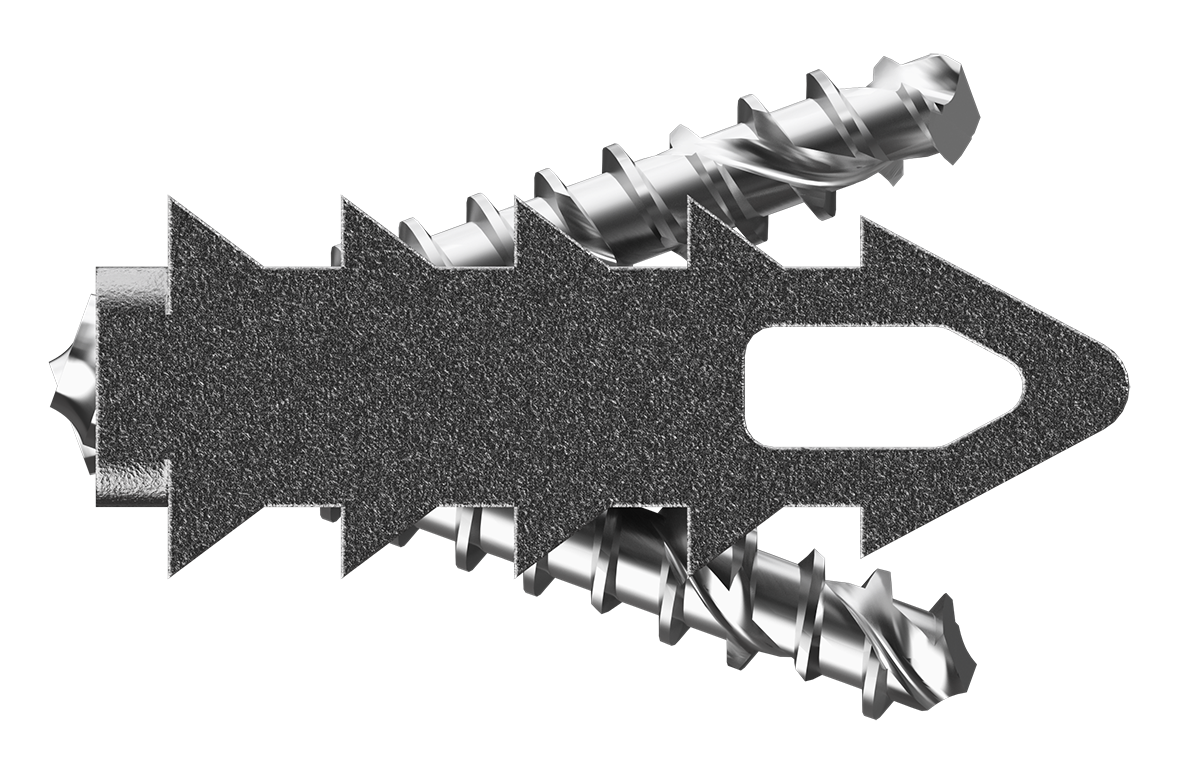
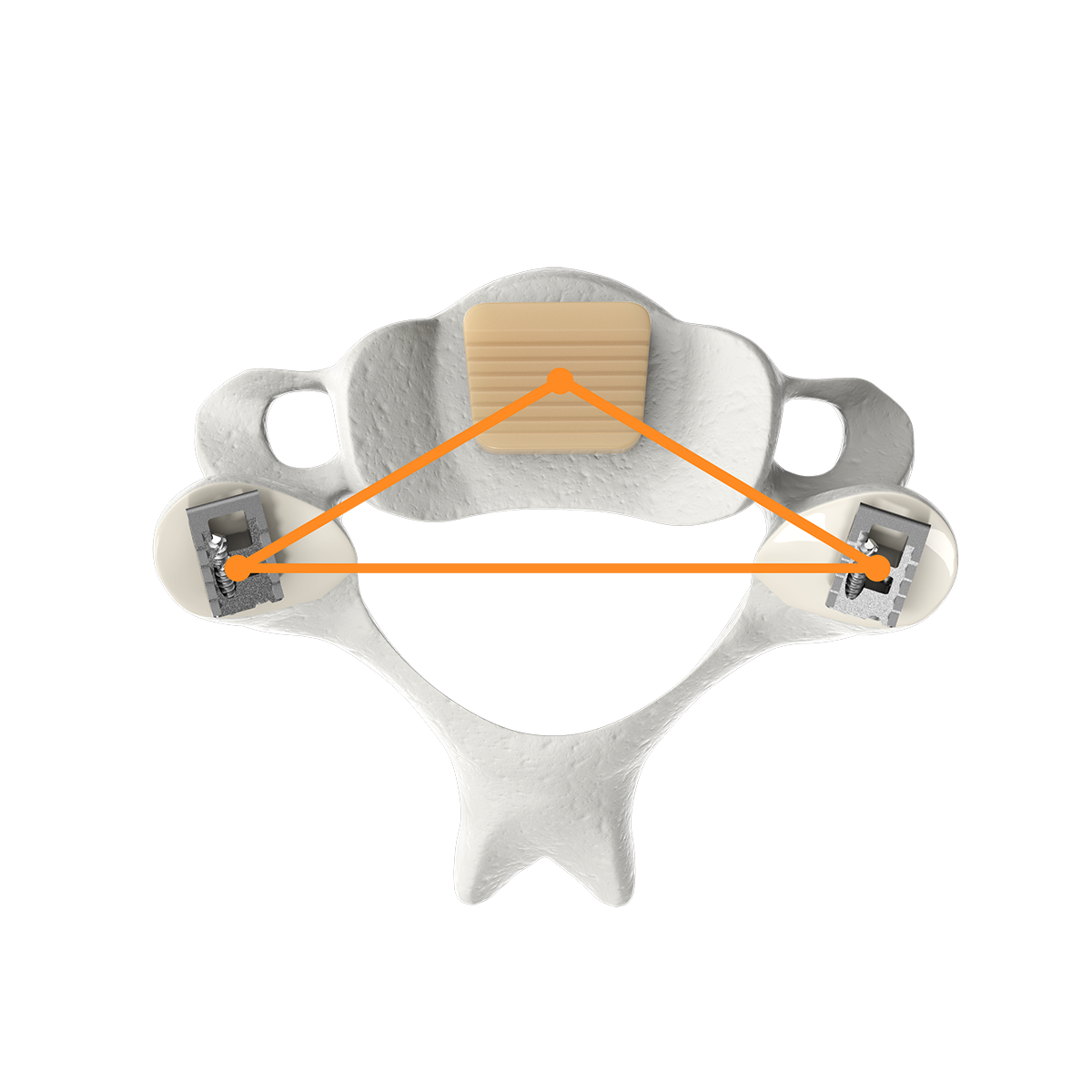
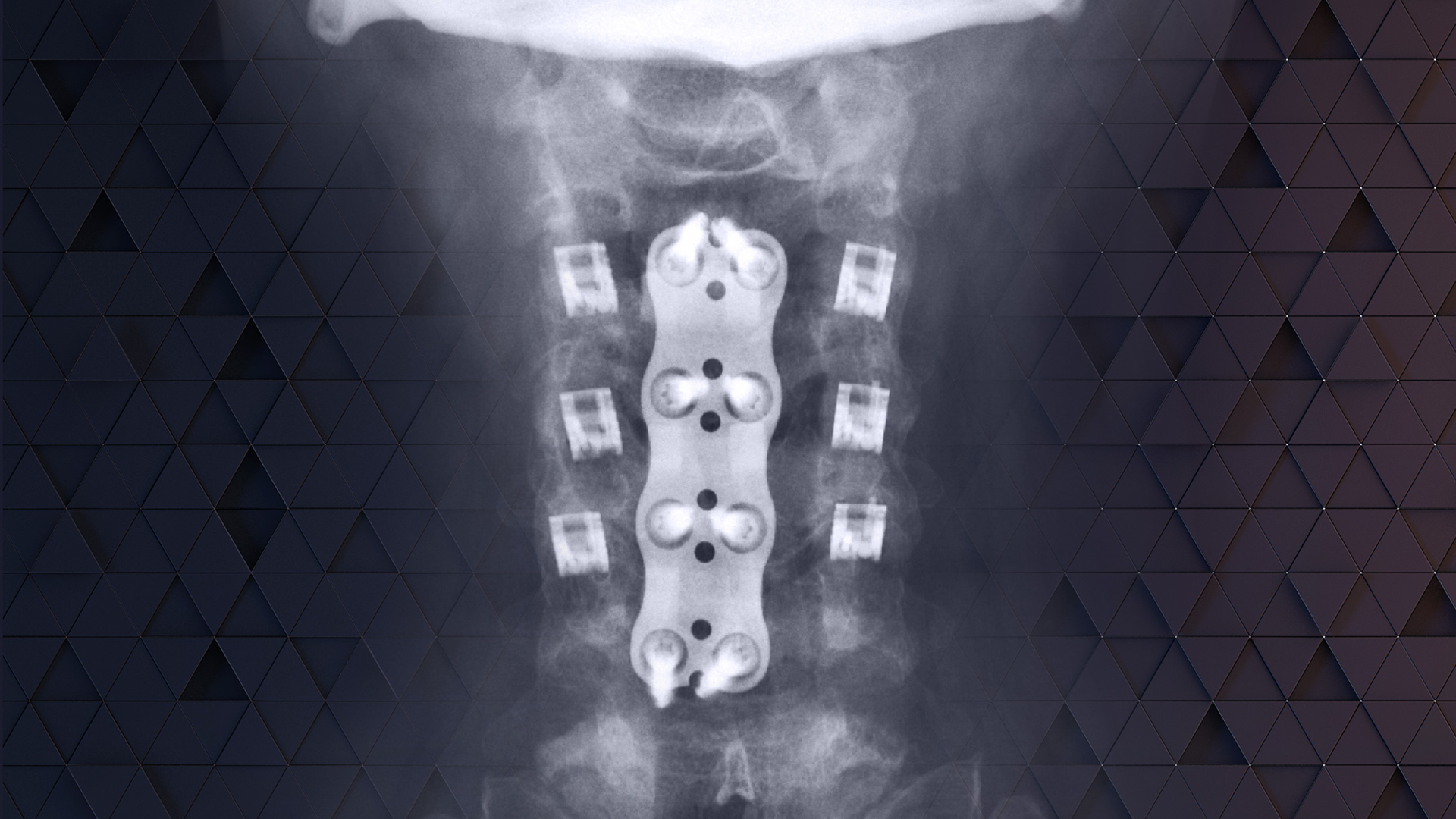
CORUS PCSS is posterior spinal instrumentation with integrated screw fixation intended to provide immobilization and stabilization of up to 3 consecutive levels of the cervical spine. PCSS is an adjunct to Posterior Cervical Fusion (PCF) and is intended to be used in combination with an Anterior Cervical Discectomy and Fusion (ACDF) at the same level(s).
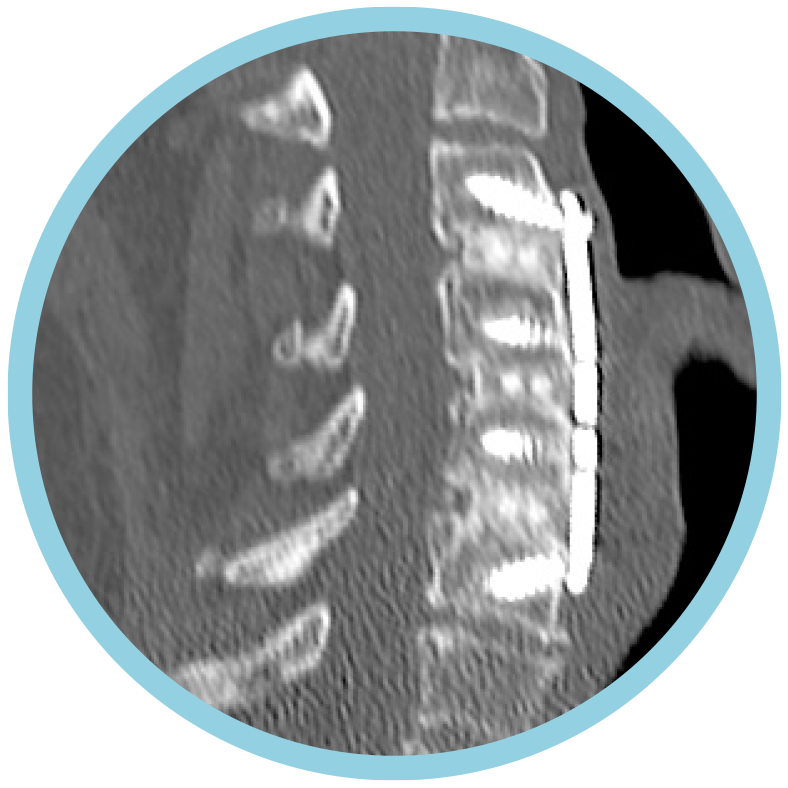
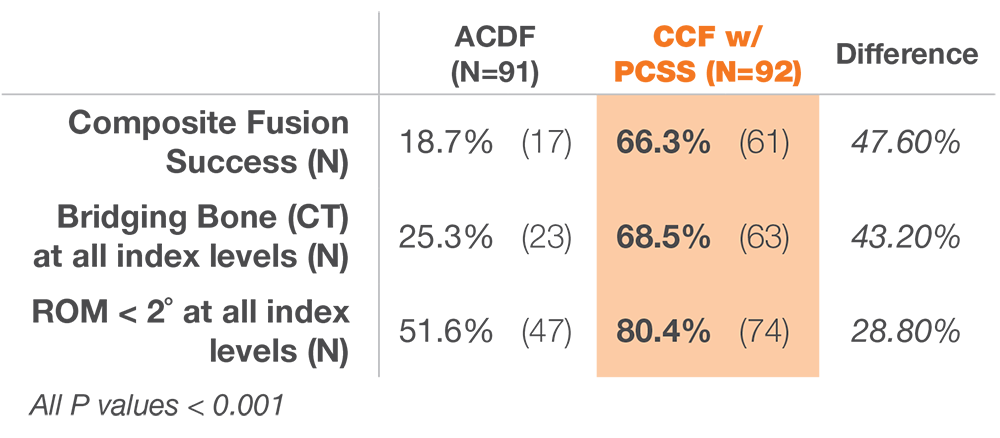
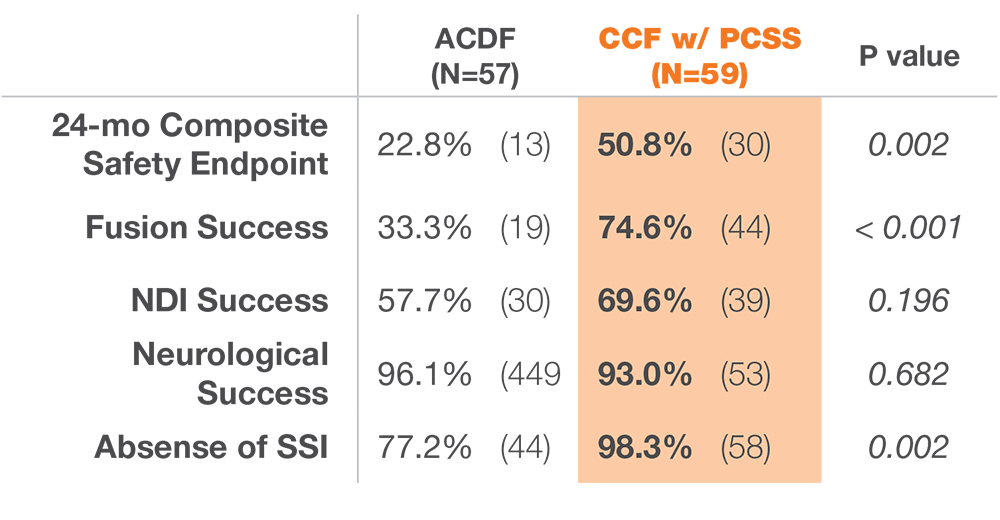
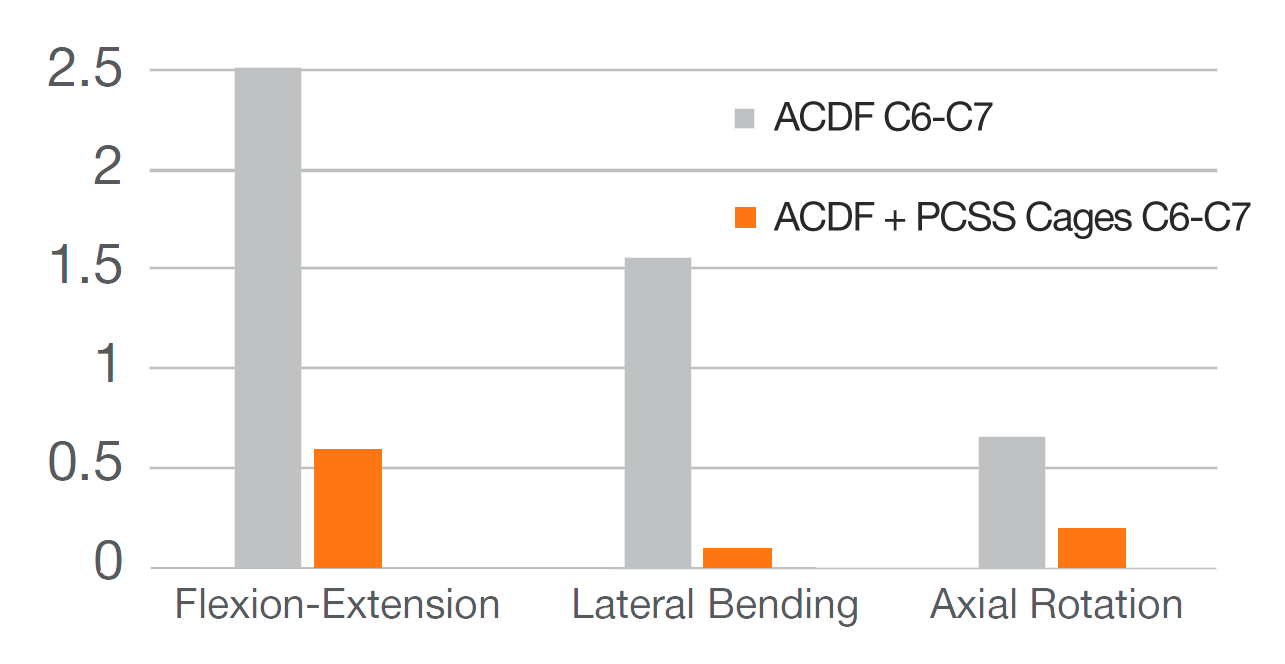
12 months after surgery, patients treated with ACDF & CORUS PCSS demonstrated a clinically superior fusion rate compared to ACDF alone.*(1)
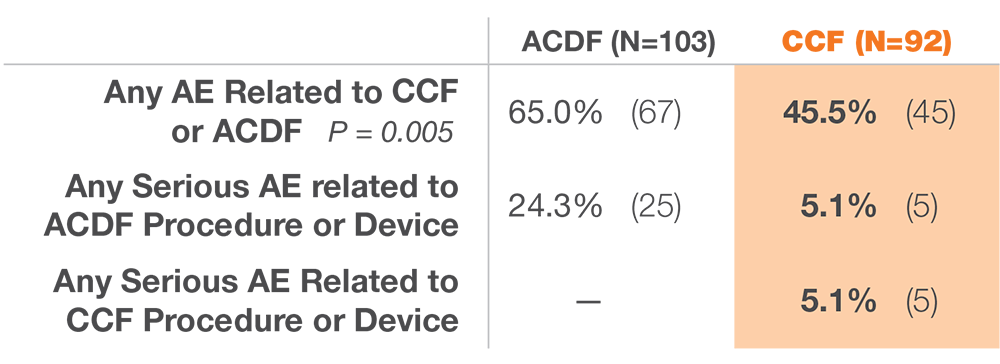
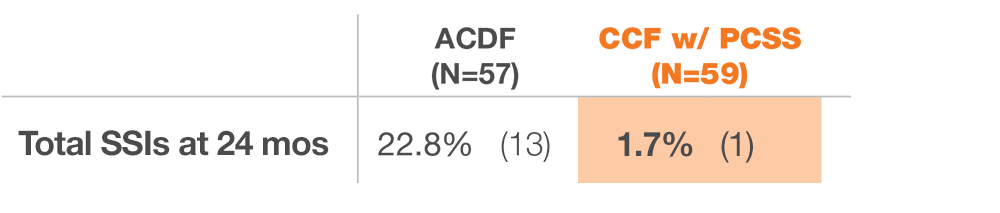


As with all medical devices, there are risks and considerations to device use. Please refer to the device labeling for a full discussion of potential risks, contraindications, warnings, precautions, and instructions for use. Rx only.
* At 12 months, the primary endpoint of fusion success demonstrated a superior fusion rate for subjects treated with ACDF and PCSS (CCF) compared to ACDF alone.(1)
† Posterior cervical fusion can be performed using open or tissue-sparing techniques. Tissue-sparing technique may require special training.
MKT-PMT-637 Rev 0
Indications for Use, CORUS™ PCSS:
CORUS™ Posterior Cervical Stabilization System (PCSS) is posterior spinal instrumentation with integrated screw fixation intended to provide immobilization and stabilization of spinal segments.
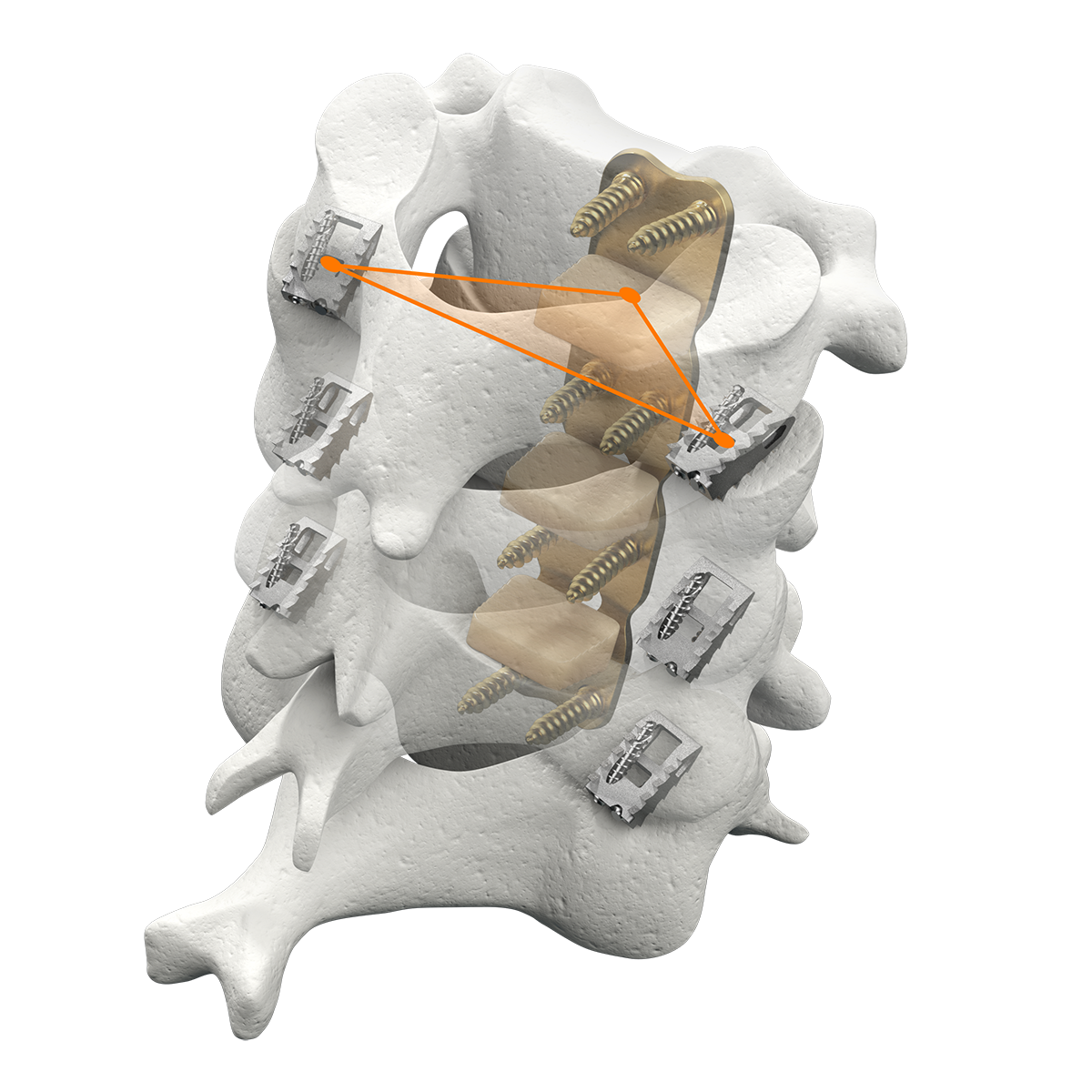
CORUS PCSS is placed through a posterior surgical approach in up to 3 consecutive levels of the cervical spine (C3-C7) and achieves bilateral facet fixation by spanning the facet interspace at each level with points of fixation at each end of the construct.
CORUS PCSS is intended as an adjunct to posterior cervical fusion (PCF) and is only intended to be used in combination with an anterior cervical discectomy and fusion (ACDF) at the same level(s).
CORUS PCSS is indicated for skeletally mature patients with degenerative disc disease (DDD). DDD is defined as radiculopathy and/or myelopathy, neck and/or arm pain of discogenic origin as confirmed by radiographic studies.
CORUS PCSS is to be used with autogenous bone and/or allogenic bone graft.
Device Description, CORUS™ PCSS:
CORUS™ PCSS is posterior spinal instrumentation with integrated screw fixation intended to provide immobilization and stabilization of spinal segments. The device is placed through a posterior surgical approach in up to 3 consecutive levels of the cervical spine (C3-C7) and achieves bilateral facet fixation by spanning the facet interspace at each level with points of fixation at each end of the construct.
The device is manufactured from medical grade titanium alloy (6Al4V) and supplied sterile for single use only with a pre-attached disposable delivery instruments. The implant is fenestrated and is to be used with autogenous bone and/or allogenic bone graft. The design incorporates “windows” through the implant to permit visualization of the graft material and, over time, formation of new bone.
CORUS™ Spinal System is used to access and prepare the site for posterior fusion.
Indications for Use, CORUS™ Spinal System-X
FOR CERVICAL FUSION: The CORUS™ Spinal System-X is a set of instruments indicated to be used to perform posterior cervical fusion in patients with cervical degenerative disc disease.
FOR LUMBAR FUSION: The CORUS™ Spinal System-X is a set of instruments indicated to be used to perform posterior lumbar fusion in patients with lumbar degenerative disc disease.
Intended Use and Indications, DiViNE Portal System
The DiViNE Portal System has applications for the creation and maintenance of an operative cavity to directly visualize an operating or examination area of the patient's body. The device may be used in all types of surgical procedures requiring retraction of tissue.
