CERVICAL
For surgeons
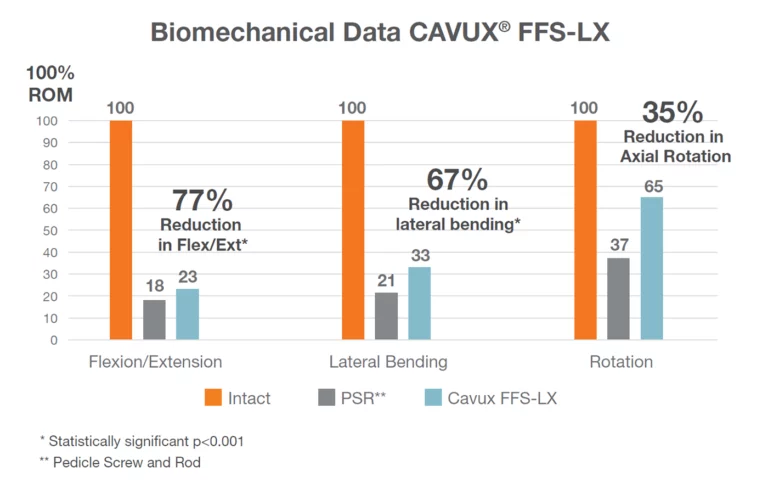
Clinical Studies



A close-up of CAVUX® FFS-LX in the lumbar spine.
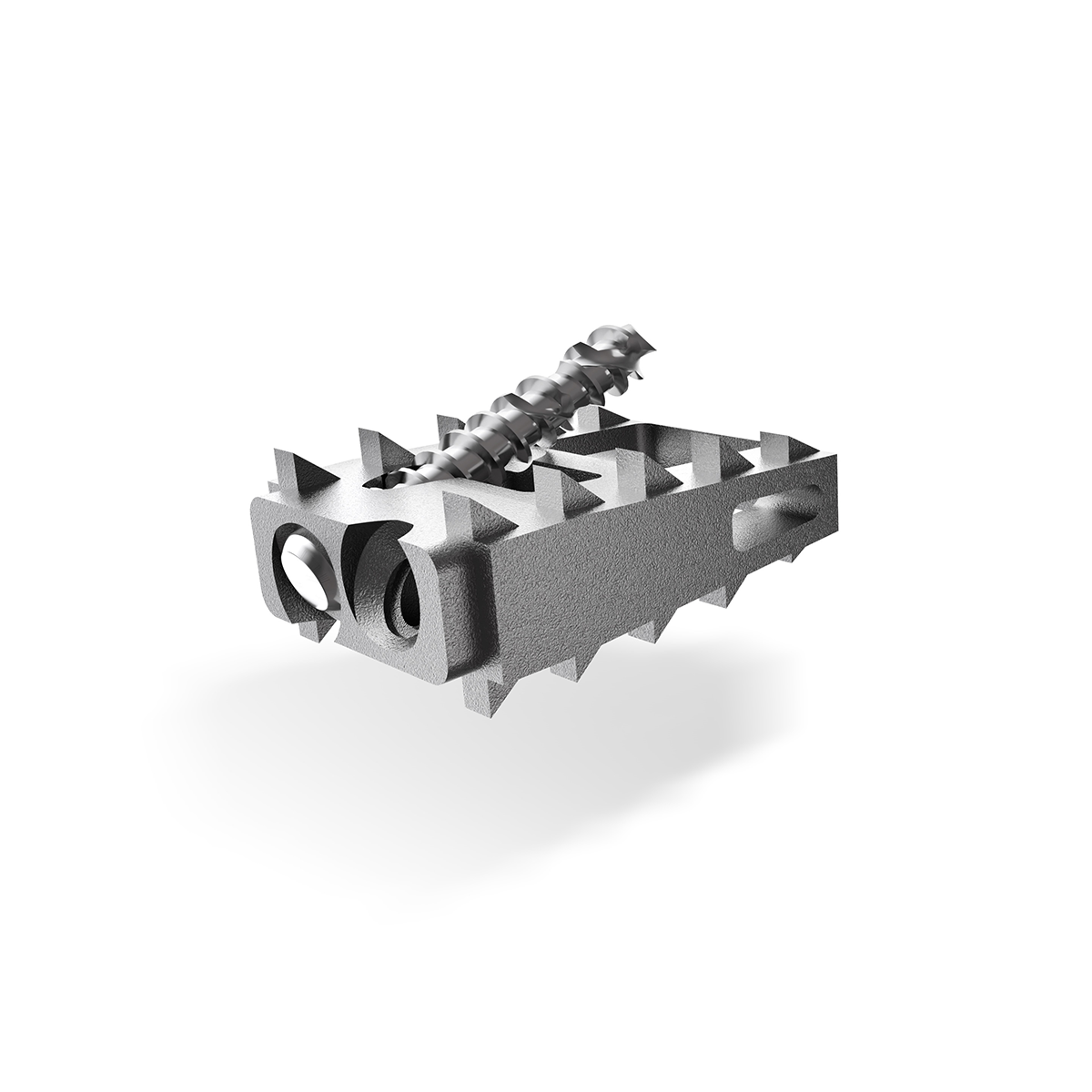
CAVUX FFS-LX spans the facet interspace with points of fixation at each end of the construct.





As with all medical devices, there are risks and considerations to device use. Please refer to the device labeling for a full discussion of potential risks, contraindications, warnings, precautions, and instructions for use.
* Incisions pictured are approximate representations of surgical access after a tissue-sparing technique. Actual incisions vary. Posterior lumbar fusion can be performed using open or tissue-sparing techniques. Tissue-sparing technique may require special training, utilize indirect visualization, and/or increase radiation exposure.
† Lumbar fusion rate defined by the FDA as less than 5 degrees (5°) of motion on dynamic, flexion-extension x-rays. The clinical study involved 57 subjects (patients).
‡ Clinically meaningful improvement in pain was achieved in 79% of clinical study subjects using a 10-point pain scale.
MKT-PMT-598 Rev 0
Indications for Use, CAVUX FFS-LX
also known as PMT Facet Fixation System, Lumbar (PMT FFS-LX)
CAVUX® Facet Fixation System, Lumbar (CAVUXFFS-LX) is an integrated construct comprised of a CAVUX Cage and an ALLY Bone Screw. PMT FFS-LX is placed bilaterally through a posterior surgical approach and spans the facet interspace with points of fixation at each end of the construct.
CAVUX FFS-LX is intended to provide temporary stabilization as an adjunct to a 1 or 2-level interbody lumbar fusion with autogenous and/or allogenic bone graft and must be accompanied with an FDA-cleared intervertebral body fusion device implanted at the same spinal level(s), and may be used with a pedicle screw and rod system implanted at the same spinal level(s).
CAVUX FFS-LX is indicated for the treatment of patients with lumbar degenerative disc disease (DDD) from L4 to S1 in skeletally mature patients who have failed conservative care.
Device Description
CAVUX Facet Fixation System, Lumbar (CAVUX FFS-LX) is composed of CAVUX® Cages and ALLY® Bone Screws, an integrated construct, manufactured from medical grade titanium alloy and supplied sterile for single use only with a pre-attached disposable delivery handle/inserter. CAVUX FFS-LX provides temporary stabilization by spanning the facet interspace with points of fixation at each end of the construct and provides fixation as an adjunct to fusion. An ALLY Bone Screw is intended to be utilized to provide additional anchoring.
Indications for Use, CORUS Spinal System-LX
The CORUS™ Spinal System-LX is a set of instruments indicated to be used to perform posterior lumbar fusion in patients with lumbar degenerative disc disease.
