CERVICAL
For surgeons
Clinical Studies

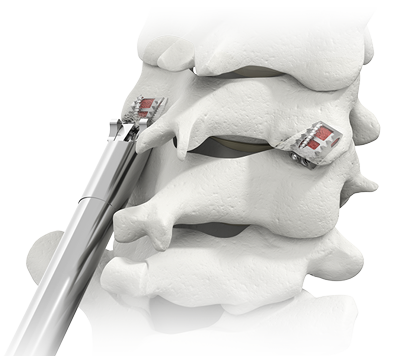
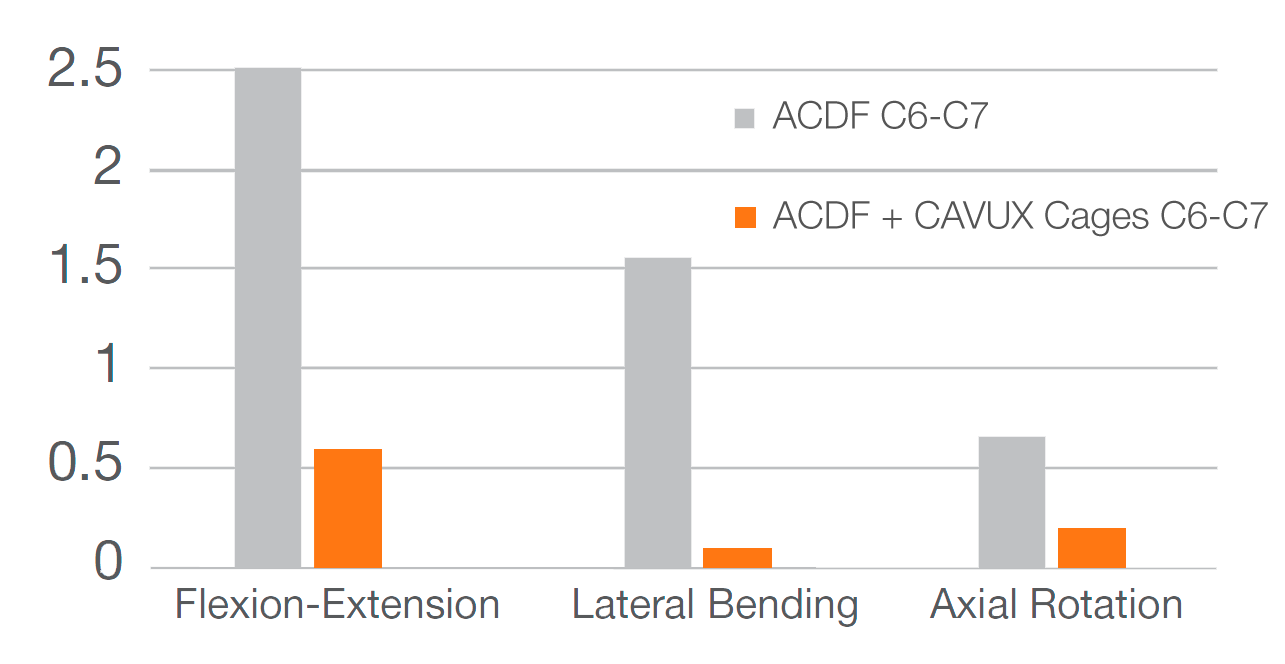
Adding CAVUX® Cervical Cages to a plated ACDF reduces range of motion and increases segmental stability.2
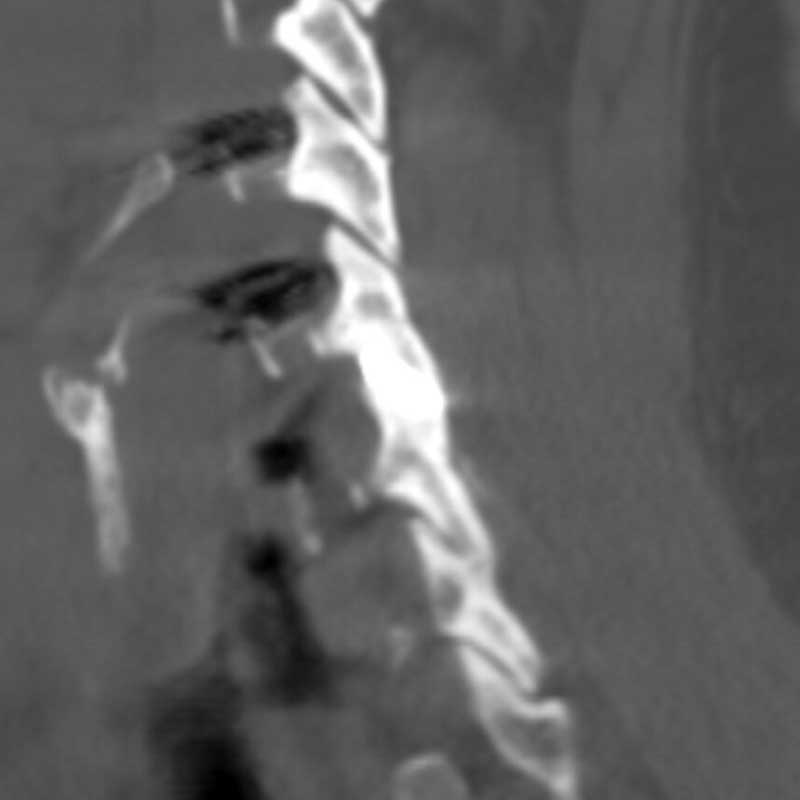
Posterior Cervical Fusion with
CORUS™ & CAVUX® FFS1
Blood Loss: 15 CC
Hospital Stay: 1.0 Day
Reoperation: 0%


As with all medical devices, there are risks and considerations to device use. Please refer to the device labeling for a full discussion of potential risks, contraindications, warnings, precautions, and instructions for use.
† Bridging trabecular bone confirmed by multiplanar CT scan read by two (2) independent radiologists.
‡ 78% of REVISE Study subjects reported pain improvement of 20% or more.
Posterior cervical fusion can be performed using and open or tissue-sparing techniques. Tissue-sparing technique may require special training, utilize indirect visualization, and/or increase radiation exposure.
MKT-PMT-522 Rev 1
* Indications for Use, CAVUX FFS
CAVUX® Facet Fixation System (CAVUX FFS) is an integrated construct comprised of a CAVUX Cage and a single ALLY Bone Screw.
CAVUX FFS is placed bilaterally through a posterior surgical approach and spans the interspace with points of fixation at each end of the construct.
CAVUX FFS is intended for temporary stabilization as an adjunct to posterior cervical fusion in skeletally mature patients.
CAVUX FFS is indicated for patients requiring a revision for an anterior pseudarthrosis at one level, from C3 to C7, with autogenous and/or allogenic bone graft.
Device Description, CAVUX FFS:
CAVUX Cages are used in conjunction with ALLY Bone Screws as an integrated construct referred to as the CAVUX Facet Fixation System “CAVUX FFS.” The device achieves facet fixation by spanning the interspace with points of fixation at each end of the construct. The device provides rigid fixation as an adjunct to fusion with the bone screw providing additional anchoring into the lateral mass. The titanium constructs are offered in various footprints and heights, and are manufactured from implant grade titanium alloy (6Al-4V ELI Titanium). CAVUX FFS is single-use only, provided sterile (gamma sterilized) with pre-attached, disposable, delivery handles.
CAVUX FFS should be implanted only using the CORUS™ Spinal System.
Indications for Use, CORUS Spinal System
FOR CERVICAL FUSION: The CORUS™ Spinal System-X is a set of instruments indicated to be used to perform posterior cervical fusion in patients with cervical degenerative disc disease.
FOR LUMBAR FUSION: The CORUS™ Spinal System-X is a set of instruments indicated to be used to perform posterior lumbar fusion in patients with lumbar degenerative disc disease.
