
| Anatomical Region |
Mean BMD Total(mgCa²⁺HA/mL) | Percent Cortical Bone(% by Volume) |
|---|---|---|
| Inferior articular process | 392.4 ± 98.4 | 51 ± 18.3 |
| Pedicle | 305.5 ± 77.0 | 36 ± 11.6 |
| Superior articular process | 306.8 ± 79.2 | 34 ± 13.9 |
| Vertebral body | 147.2 ± 26.9 | 10.9 ± 5.2 |
Abbreviations: BMD, bone mineral density; Ca²⁺HA, calcium hydroxyapatite

Our Mission is to establish Circumferential Cervical Fusion (CCF) as the standard of care for high-risk patients.
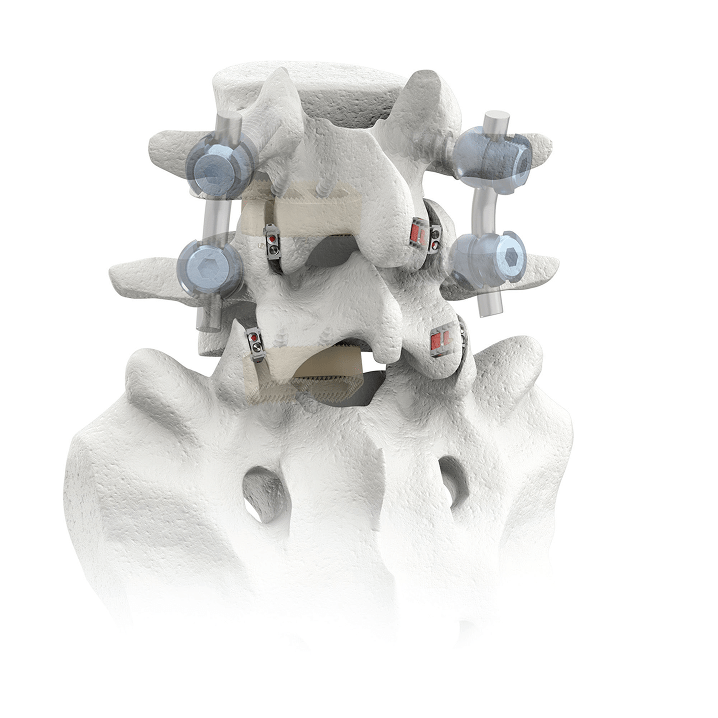
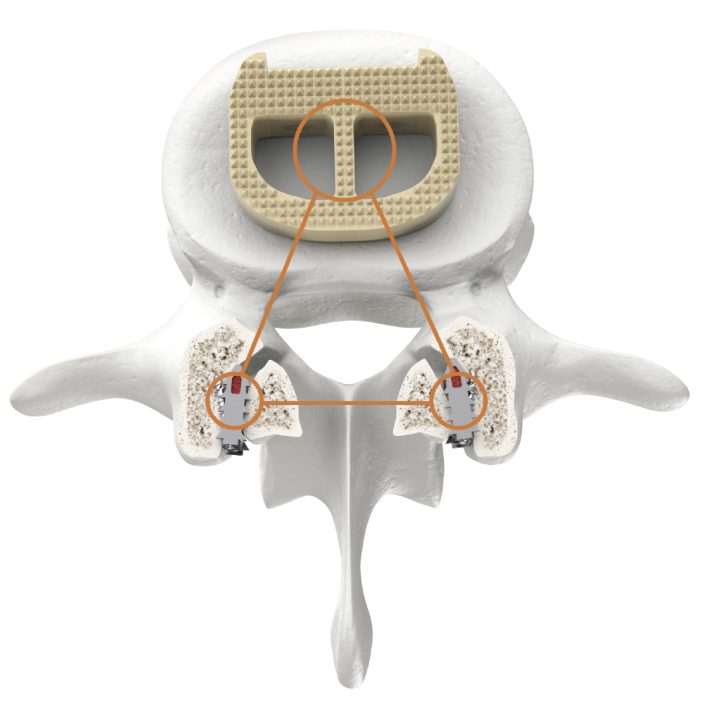
CORUS-LX Implant is posterior spinal instrumentation with integrated screw fixation intended to provide immobilization and stabilization of spinal segments. CORUS-LX is placed bilaterally through a posterior surgical approach and spans the facet interspace with points of fixation at each end of the construct.
CORUS-LX is intended to provide temporary stabilization as an adjunct to a 1 or 2 level interbody lumbar fusion with autogenous and/or allogenic bone graft and must be accompanied with an FDA cleared intervertebral body fusion device implanted at the same spinal level(s), and may be used with a pedicle screw and rod system implanted at the same spinal level(s).
CORUS-LX is indicated for the treatment of patients with lumbar degenerative disc disease (DOD) from L4 to S1 in skeletally mature patients who have failed conservative care.
