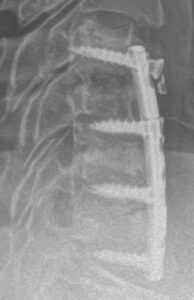
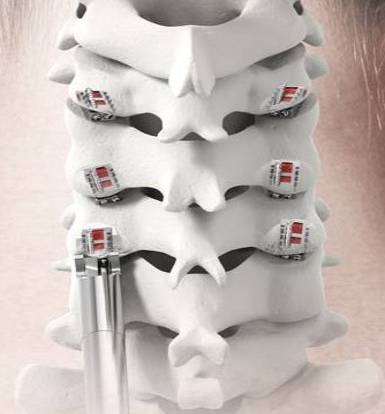
CERVICAL
For surgeons
Clinical Studies
| Design | Multicenter, Prospective, Randomized, Controlled Trial |
| Total Patients | N=227 randomized |
| Control Group | ACDF with plate |
| Test Group | CCF (Circumferential Cervical Fusion): Plated ACDF plus PCF with PCSS |
| Target Population | Subjects requiring 3 level fusion for the treatment of symptomatic DDD |
| Primary Endpoint at 12 months | Fusion Success Superiority – Bridging trabecular bone across endplates & – Less than 2° angular motion in flexion/extension |
| Secondary Endpoint at 24 months | Composite Success Noninferiority – Fusion success – Improvement in Neck Disability Index (NDI) – Neurological success – Absence of secondary surgical interventions (SSI) |
| Follow-ups | 6 weeks; 3, 6, 12 & 24 months |
| Study Hypothesis | Circumferential fusion improves outcomes for patients undergoing 3-level procedures |