CERVICAL
For surgeons
Clinical Studies
PLEASANTON, Calif., May 11, 2023 /PRNewswire/ -- Providence Medical Technology, Inc. (PMT), a leader in medical device innovation for spine surgery, today announced the completion of enrollment in its FUSE Study — a prospective, multicenter, randomized, Investigational Device Exemption (IDE) clinical study evaluating the safety and effectiveness of its Posterior Cervical Stabilization System (PCSS) in 3-level cervical fusion patients. The study enrolled approximately 230 patients across 18 leading spinal surgery sites in the United States to evaluate PCSS in combination with Anterior Cervical Discectomy and Fusion (ACDF) compared to ACDF alone in a superiority study.
The study began in May 2020 and is designed to evaluate Circumferential Cervical Fusion (CCF) in high-risk cervical fusion patients by producing level-1 clinical evidence. The study includes patients at risk for failure of a standard ACDF fusion procedure due to risk factors such as 3-level disease, smoking, advanced age, and diabetes. While a traditional ACDF procedure is generally associated with positive outcomes in otherwise healthy patients, the success rate for these high-risk patients is significantly less favorable. Of the over 300,000 anterior cervical fusion procedures performed annually, roughly 30% involve patients with significant risk factors for nonunion. This study is investigating whether these patients could benefit from a tissue-sparing posterior cervical fusion as an adjunct to the ACDF.
The first comparative analysis for fusion and other clinical outcomes will occur when 100 patients reach two years of follow-up. The 100th patient is scheduled to return for their two-year follow-up visit at the end of 2023. View Release
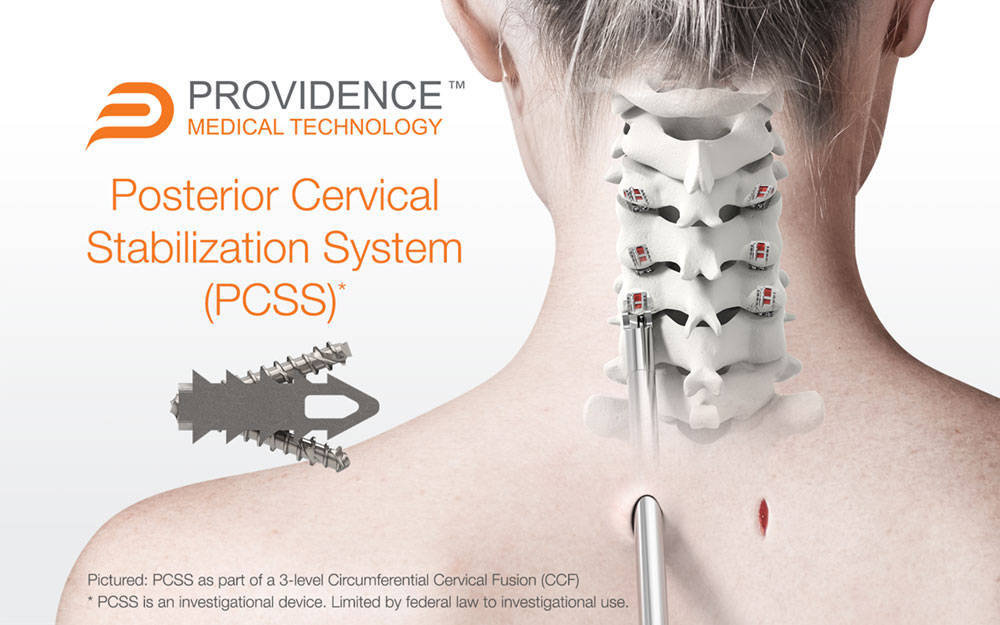
PCSS is an investigational implant system composed of non-segmental instrumentation with integrated screw fixation designed to provide immobilization and stabilization of spinal segments. PCSS is designed to achieve bilateral facet fixation at each level by spanning the interspace with points of fixation at each end of the construct.
