CAVUX FFS-LX Achieved 96% Fusion Rate Verified by Independent Core Imaging Lab in Recent Clinical Study
Pleasanton CA, January 10, 2024 / PRNewswire/ -- Providence Medical Technology, Inc. (PMT), a medical device innovator focused on improving surgical outcomes for high-risk spine surgery patients, announced that the U.S. Food and Drug Administration (FDA) has cleared its CAVUX® FFS-LX: Lumbar Facet Fixation System for use in lumbar spinal fusion surgery.
CAVUX® FFS-LX is a novel integrated cage and screw system that is implanted bilaterally in the facet joints to treat lumbar degenerative disc disease (DDD). The implant spans the facet interspace with points of fixation at each end of the construct to offer additional stabilization for 1- or 2-level lumbar interbody fusion. CAVUX® FFS-LX may be used with or without pedicle screws and rods and is implanted using the company’s CORUS™ Spinal System-LX tissue-sparing access and spinal fusion system.
The clearance marks the company's expansion into the $2 billion lumbar spine market characterized by a higher surgical failure rate than in the cervical spine. Patients who fail to fuse after a lumbar spine fusion surgery face a substantial risk of added complications, suffering, and costly revision procedures. CAVUX® FFS-LX was designed to offer increased stabilization following lumbar fusion procedures in order to increase fusion rates and reduce future complications and reoperations.
The clearance was supported by clinical study data demonstrating a strong safety and efficacy profile. Summary clinical study data reviewed by the FDA in considering the clearance included:
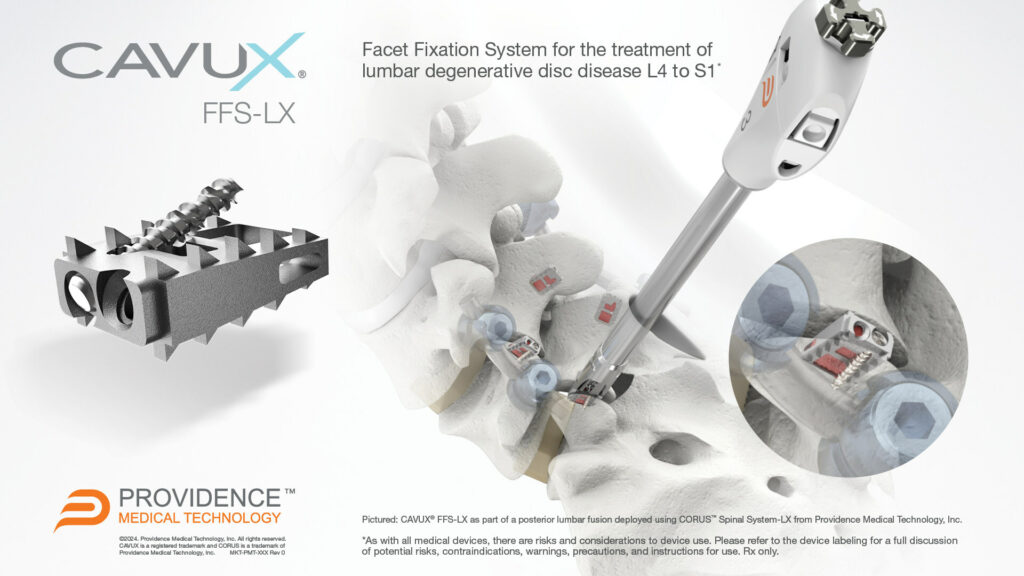
CAVUX® FFS-LX (Facet Fixation System, Lumbar) is a novel integrated cage and screw system to treat lumbar degenerative disc disease that is now FDA-cleared for use in lumbar spinal fusion surgery.
